The litigation surrounding GLP-1 receptor agonists (GLP-1 RA), including Ozempic and Wegovy, represents a significant chapter in the ongoing scrutiny of pharmaceutical safety and marketing ethics. Stemming from allegations that severe gastrointestinal side effects were downplayed or omitted by the drugs’ manufacturers, MDL No. 3094 underscores a critical legal battle. This comprehensive document integrates FDA’s Adverse Event Reporting System (FAERS) data with proprietary insights from Blue Sky Legal’s targeted campaign, aiming to reveal the depth and breadth of the potential plaintiff pool. By furnishing mass tort attorneys with an exhaustive analysis, this overview seeks to illuminate the extensive reach of the litigation, highlighting the substantial number of individuals potentially impacted.
The trajectory of GLP-1 RA medications like Ozempic and Wegovy has been marked by their clinical success but shadowed by legal controversies. This document, prepared by Blue Sky Legal, aims to dissect the scale of potential plaintiffs arising from alleged inadequate warnings about the drugs’ serious side effects. Leveraging both public and proprietary data sources, we offer a holistic view of the litigation landscape, focusing on the expansive number of affected individuals.
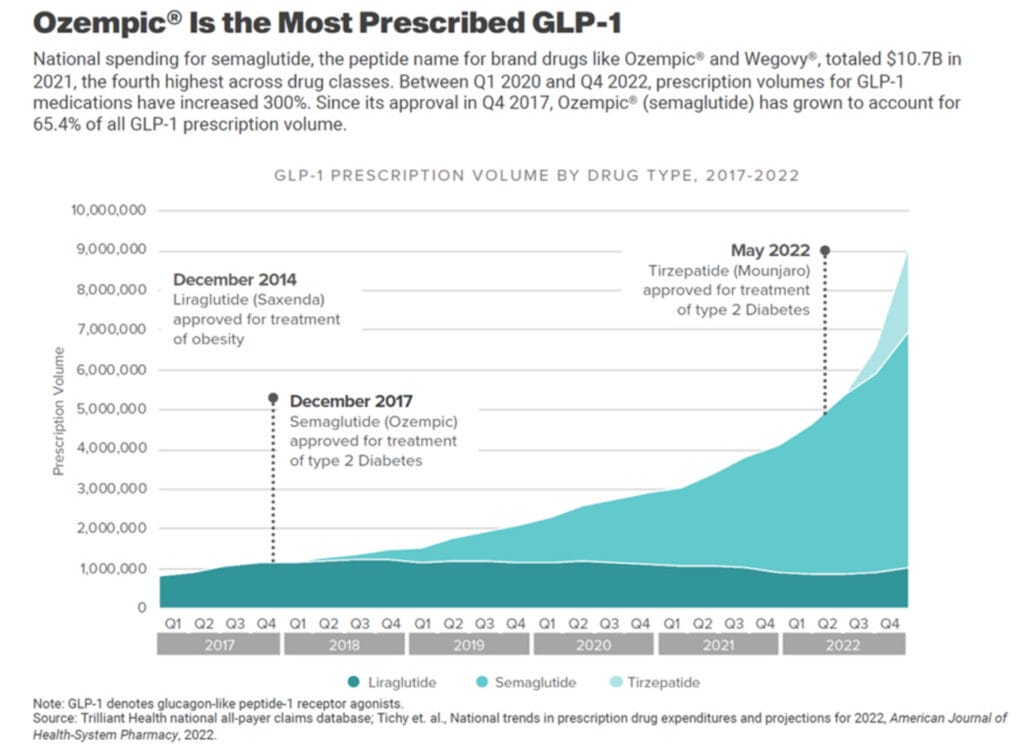
The Emergence of GLP-1 RA Litigation
MDL No. 3094 reflects a growing discord between pharmaceutical innovation and consumer safety, with plaintiffs alleging that the manufacturers of GLP-1 RA drugs failed to sufficiently inform about risks of severe gastrointestinal complications. These allegations point to a larger narrative of potentially misleading marketing practices prioritizing drug benefits over transparent risk communication.
Methodological Synthesis: Combining FAERS with Blue Sky Legal Campaign Insights
This case overview harnesses a dual-source approach to data analysis, blending FAERS adverse event reporting with direct feedback and reports from individuals engaged through Blue Sky Legal’s proprietary campaign. This methodology not only enriches the data pool but also ensures a multifaceted understanding of the litigation’s potential scale.
In-Depth Analysis of Potential Claimants
Table 1: Detailed Summary of Adverse Events from FAERS (2014-2024)
| Serious Outcome | Number of Cases | Estimated Reporting Rate (10%) | Estimated Reporting Rate (5%) |
| Died | 131 | 1,310 | 2,620 |
| Disabled | 214 | 2,140 | 4,280 |
| Hospitalized | 2,375 | 23,750 | 47,500 |
| Total | 2,720 | 27,200 | 54,400 |
This table extrapolates the reported cases to estimate the full scope of serious adverse events, underscoring the potential underreporting of such incidents.
Table 2: Comprehensive Distribution of Claimant Injuries from Blue Sky Legal Campaign
| Injury Type | Instances | Percentage of Total Injuries |
| Received Medical Care for Vomiting for 4+ Weeks | 55 | 36% |
| Severe/Permanent Stomach Paralysis (Gastroparesis) | 32 | 21% |
| Ileus or Bowel Obstruction | 24 | 16% |
| Gallbladder Removal (Pre-March 2022) | 20 | 13% |
| DVT or Related Injuries (Age ≤ 60) | 9 | 6% |
This distribution provides a snapshot of the most common injuries reported, highlighting the gastrointestinal focus of the adverse events.
Timeline and Analysis of GLP-1 RA Litigation Developments
- 2014-2024: FAERS data collection period, capturing a decade of adverse event reporting.
- February 25, 2023: Latest comprehensive update from FAERS, marking over 5,316 reported serious adverse events.
- 2023: Official commencement of MDL No. 3094, with plaintiffs seeking accountability for alleged misrepresentations of drug safety.
- February 2024: Fifty-five lawsuits alleging severe gastrointestinal injuries from the use of GLP-1 RA drugs, including Ozempic, Wegovy, Rybelsus, Mounjaro, and Trulicity, have been centralized in the Eastern District of Pennsylvania, with U.S. District Judge Gene E.K. Pratter
- Ongoing: Blue Sky Legal’s campaign continues to gather and analyze data from affected individuals, further refining the understanding of the claimant pool’s size and composition.
Strategic Implications for Mass Tort Attorneys
The expansive potential plaintiff pool highlighted in this analysis necessitates a calibrated, well-informed legal strategy. Mass tort attorneys are called upon to navigate a complex landscape, balancing the nuances of individual adverse events against the broader patterns of potential negligence and misrepresentation by drug manufacturers.
Expanding the Dialogue: Community Impact and Legal Perspectives
Beyond the immediate legal considerations, this case overview prompts a broader discussion on the impact of pharmaceutical practices on community health and the role of legal systems in ensuring drug safety and accountability. It invites stakeholders across the spectrum, from healthcare professionals to regulatory agencies, to reevaluate the mechanisms of drug approval, marketing, and post-market surveillance.
The potential plaintiff pool in the GLP-1 RA litigation, as revealed through a detailed synthesis of FAERS and proprietary campaign data, represents a significant and underappreciated aspect of this legal battle. This document underscores the importance of a multidimensional approach to legal strategy, informed by a comprehensive analysis of available data. For mass tort attorneys, this overview not only provides a foundational understanding of the scope of claims but also highlights the critical need for strategic planning in addressing the extensive harm reported by affected individuals. Through collective legal efforts, there exists an opportunity to advocate for enhanced drug safety and transparency, ensuring that the benefits of pharmaceutical innovations do not come at the cost of patient well-being.